By Kim Smiley
Avaira Toric contact lenses, manufactured by CooperVision Inc, were voluntary recalled on August 19, 2011. The recall occurred after dozens of consumers complained about eye problems including impaired vision, eye pain and torn corneas. According to a company statement, a manufacturing error resulted in a silicone oil residue on some contacts. More than 8 million lenses were affected worldwide by the recall, but only about 780,000 of these contact lenses were distributed in the USA.
The company has received a large amount of negative media attention following the recall. News articles and blogs have claimed that CooperVision was purposely downplaying the recall, resulting in many consumers being unaware that their lenses had been recalled. Unaware of the potential danger, consumers continued to wear their lenses and continued to have eye problems as a result. The FDA publicly threatened to independently inform consumers of the risk associated with these contact lenses if the manufacturer didn’t better publicize the recall.
 Following the media attention , the company has increased efforts to notify consumers about the recall. The FDA has also posted a notice on their website of this recall, identifying it as a Class I recall, the most serious class of recall. The FDA has said that the company’s actions are now consistent with what would be expected with a Class I recall.
Following the media attention , the company has increased efforts to notify consumers about the recall. The FDA has also posted a notice on their website of this recall, identifying it as a Class I recall, the most serious class of recall. The FDA has said that the company’s actions are now consistent with what would be expected with a Class I recall.
This example is a good illustration that the execution of a recall is a very important thing. If consumers view a recall as slow to happen or badly executed, they will probably be less likely to trust a company in the future. Ideally, a recall should be executed so that consumers are left with the feeling that a company did the right thing as quickly as possible.
If you are concerned that you might have recalled lenses, you can visit this website to check. If your lenses are recall, you are asked to remove them immediately and return them to the point of purchase.
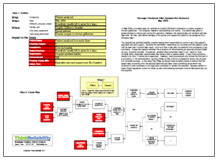
Click on “Download PDF” above to view an outline and initial Cause Map of this example.