Recent research has shown that in-hospital mortality for patients who acquire an infection in the hospital increases from 4.5% to 18.5%. Hospital-acquired infections are infections obtained while a patient is hospitalized. The three main hospital-acquired infections (or HAIs) are bloodstream infections (28% of HAIs), pneumonia (21%) and urinary tract infections (15%).
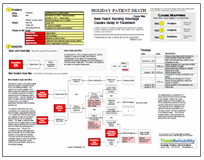
Not only does an HAI increase the mortality rate, it has other impacts as well. We can look at these impacts, and their causes, in a root cause analysis demonstrated visually as a Cause Map. For the purpose of this root cause analysis, we will limit our investigation to HAIs that occur during hospitalization in an intensive care unit (ICU). We begin with determining the other impacts to the goals. The patient safety goal is impacted due to the increase in mortality. The organization goal is impacted because many insurers (including Medicare and Medicaid) will not reimburse for some infections obtained during hospitalization. Additional treatment is required to treat the infection, resulting in an impact to the patient services goal. The treatment for these infections normally results in an increased stay in the ICU (from an average of 8.1 days to 15.8 days), at a cost of $16,000. It is estimated that 26.7% of all ICU stays result in at least one HAI.
 Beginning with the impacted patient safety goal, we can ask “Why” questions to demonstrate the cause-and-effect relationships leading to the increase in mortality. Increased mortality is due to the acquiring of an HAI. HAIs result from the exposure to a pathogen and frequently occur in the ICU partially due to the increased risk of infection due to the underlying condition for which the patient is in the ICU. There are two types of pathogens to which patients can be exposed: endogenous (essentially, from the patient’s own body) and exogenous (from visitors, healthcare providers, equipment, the environment, etc). HAIs are highly related to the use of invasive support measures, which provide a path for either kind of pathogen directly into the patient’s body. Specifically, the use of a central intravenous line is cited in 91% of bloodstream infections, mechanical ventilation is cited in 95% of hospital-acquired pneumonias, and urinary catheters are cited in 77% of urinary tract infection.
Beginning with the impacted patient safety goal, we can ask “Why” questions to demonstrate the cause-and-effect relationships leading to the increase in mortality. Increased mortality is due to the acquiring of an HAI. HAIs result from the exposure to a pathogen and frequently occur in the ICU partially due to the increased risk of infection due to the underlying condition for which the patient is in the ICU. There are two types of pathogens to which patients can be exposed: endogenous (essentially, from the patient’s own body) and exogenous (from visitors, healthcare providers, equipment, the environment, etc). HAIs are highly related to the use of invasive support measures, which provide a path for either kind of pathogen directly into the patient’s body. Specifically, the use of a central intravenous line is cited in 91% of bloodstream infections, mechanical ventilation is cited in 95% of hospital-acquired pneumonias, and urinary catheters are cited in 77% of urinary tract infection.
Because these invasive support measures are generally required for patient care, it’s difficult to see how these infections can be reduced. However, some programs have been shown to substantially reduce HAIs – and the cost associated with them – by improving the culture of safety and compliance with preventive methods. One such program in Michigan has reduced the rate of bloodstream infections associated with central lines from 7.7 to 1.3 per 1,000 catheter days. Even without a dedicated safety program, insisting on hand washing and proper cleanliness procedures during the insertion, checking, and removal of invasive support measures can reduce the risk of HAIs. Additionally, because the use of invasive support measures is so strongly correlated to HAIs, removal of these measures as soon as possible can also reduce the risk.
To view the Outline and Cause Map, please click “Download PDF” above. Click here to read more about hospital-acquired conditions. Or click here to read more about the latest research.