By Kim Smiley
Shortage of a lifesaving cancer drug, methotrexate, has hospitals scrambling to find enough drugs to treat patients. Methotrexate has long been a treatment for acute lymphoblastic leukemia, or ALL, and a type of bone cancer called osteogenic sarcoma. A particular form of methotrexate without alcohol-based preservatives is needed to treat ALL because a high dose must be injected directly into the spines of patients and preservatives can be toxic and cause paralysi at such a high dose. With treatment, ALL can be cured more than 90 percent of the time. What makes this drug storage particularly heart breaking is that ALL most often strikes between ages 2 to 5. If hospitals don’t have adequate supplies of preservative free methotrexate, children will die from a disease that is largely curable.
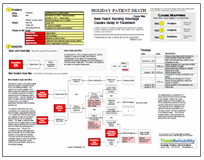
This issue can be analyzed by building a Cause Map, an intuitive, visual root cause analysis. A Cause Map is built by determining the impact to the overall goals and then asking “why” questions to add causes that contributed to the issue and show the cause-and-effect relationships between the causes. In this example, the safety goal is clearly impacted because there is a risk of patient death.
 To begin adding causes to the Cause Map, we could ask why that is true. Patients may die because they have cancer, the doctors may not be able to treat the cancer and the cancer is fatal if untreated. Why might the doctors be unable to treat the cancer? There is a shortage of the required medication because the plant that was the primary supplier for US is shut down. The plant is voluntarily shut down so that significant manufacturing and quality issues can be addressed. In order to understand the issues, it is also worth asking why one plant manufactured so much of the supply of methotrexate. As much detail as necessary can be added to the Cause Map. Once the Cause Map is built, the information can be used to brain storm solutions and determine which should be implemented. To view a high level Cause Map of this issue, click on “Download PDF” above.
To begin adding causes to the Cause Map, we could ask why that is true. Patients may die because they have cancer, the doctors may not be able to treat the cancer and the cancer is fatal if untreated. Why might the doctors be unable to treat the cancer? There is a shortage of the required medication because the plant that was the primary supplier for US is shut down. The plant is voluntarily shut down so that significant manufacturing and quality issues can be addressed. In order to understand the issues, it is also worth asking why one plant manufactured so much of the supply of methotrexate. As much detail as necessary can be added to the Cause Map. Once the Cause Map is built, the information can be used to brain storm solutions and determine which should be implemented. To view a high level Cause Map of this issue, click on “Download PDF” above.
In this example, the FDA is currently negotiating with five plants that are approved to manufacture methotrexate to increase their production of the drug. In the meantime, the plant that was shut down has worked with the FDA to allow distribution of some of the methotrexate that was manufactured, but not shipped prior to the shutdown. Hospitals still have a smaller supply of methotrexate than would be desired, but all patients’ needs are currently being met.