In November 11, 2005, a woman in labor checked in to an Army Medical Center in Hawaii. The mother was placed in the care of a second-year medical resident. The fetus showed signs of distress throughout the day, and “took a turn for the worse” at approximately 5:00 p.m. However, the child was not delivered until nearly 6:00 p.m. when the fetus was “almost dead”. The baby was born with the umbilical cord wrapped around her neck and was turned over to another team. On this team, a first-year intern placed an oxygen tube incorrectly, resulting in oxygen being delivered to the baby’s stomach instead of her lungs for approximately 40 minutes. The child now has severe brain damage and the family was awarded a $11 M settlement for her care. This is the fourth large settlement this hospital has made relating to errors made from 2003-2007, with an average of $11M per year to settle the lawsuits.
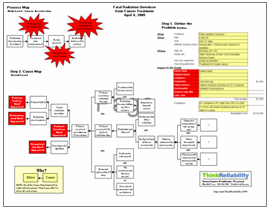
 There are several impacts to the goals of the medical center; namely, the impact to patient safety resulting from the injury to the child, the impact to the organizational goals from the settlement, the impact to patient services from the delay to the birth, and the impact to the time and labor goal for additional work required as a result of the issues with the child. Our analysis begins with these impacts to the goals.
There are several impacts to the goals of the medical center; namely, the impact to patient safety resulting from the injury to the child, the impact to the organizational goals from the settlement, the impact to patient services from the delay to the birth, and the impact to the time and labor goal for additional work required as a result of the issues with the child. Our analysis begins with these impacts to the goals.
The injury to the child was caused by a lack of oxygen, caused in part from insufficient oxygen before the birth and in part because of insufficient oxygen after the birth. The baby did not have sufficient oxygen before birth because the umbilical cord was wrapped around her neck and her birth was delayed, due to a “lack of communication” between the second-year resident and her supervisor who were charged with the mother’s care. More detail on this lack of communication is not currently available; however, from the perspective of the medical center involved, this is a key place where more detail needs to be added to the Cause Map once it is available.
The baby had insufficient oxygen after birth because the oxygen tube placed to increase her oxygen levels was feeding into her stomach rather than her lungs. The tube was misplaced by a first-year intern who was being insufficiently supervised. (Note that the reports don’t say this anywhere, but if you have an intern under supervision who places a tube incorrectly, you can conclude that the supervision was insufficient.) Note this is another area that requires more detail for the investigation to be complete in order to find effective solutions. As with any investigation the level of detail in the analysis is based on the impact of the incident on the organization’s overall goals. Because of the extremely high impact on patient safety, the analysis for this issue should be quite detailed.