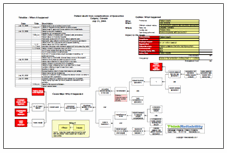
In May 2008, a fourteen-year-old entered an English Children’s Hospital for a routine surgery to remove gallstones. The recovery, however, was anything but routine. The patient was given a spinal epidural to reduce pain during the operation; however, the epidural was not removed until two days later. By then, permanent damage of the spinal cord caused the patient to be paralyzed from the waist down.
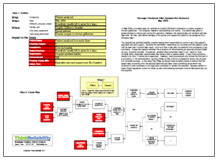 The hospital has admitted liability, possibly leaving them responsible for some or all of the patient’s specialist care and support. Because the anesthetic needle was not removed until the patient’s body until far later than it should have been – and more than a day after the patient’s first complaints of leg numbness – it begs the question whether the procedure for administering an epidural included follow-up care, including removal. Procedures – whether they are written down or not – exist for most complex tasks, especially medical tasks that involve risks to patient safety. If use of the procedure results in an error, it should be re-examined. However, many procedures only include the first part of a procedure, or the administration, ignoring follow-up that must be completed to ensure the process is a complete success. In this case, that follow-up should have included checks to ensure that the patient was recovering from the epidural (which would have noted something amiss when she continued to feel numbness in her legs) and a schedule to remove the epidural. Because neither of these things happened, a plan for follow-up after administering epidurals must be developed and put into practice.
The hospital has admitted liability, possibly leaving them responsible for some or all of the patient’s specialist care and support. Because the anesthetic needle was not removed until the patient’s body until far later than it should have been – and more than a day after the patient’s first complaints of leg numbness – it begs the question whether the procedure for administering an epidural included follow-up care, including removal. Procedures – whether they are written down or not – exist for most complex tasks, especially medical tasks that involve risks to patient safety. If use of the procedure results in an error, it should be re-examined. However, many procedures only include the first part of a procedure, or the administration, ignoring follow-up that must be completed to ensure the process is a complete success. In this case, that follow-up should have included checks to ensure that the patient was recovering from the epidural (which would have noted something amiss when she continued to feel numbness in her legs) and a schedule to remove the epidural. Because neither of these things happened, a plan for follow-up after administering epidurals must be developed and put into practice.
To view the Outline and Cause Map, please click “Download PDF” above.