By Kim Smiley
In recent years, keratin-based hair products have become increasingly popular. They smooth hair and many rave over their effective de-frizzing abilities. These products are expensive, but are consumers paying an even higher price for beautiful hair?
Health concerns about the use of keratin-based hair products have been reported multiple times over the past several years. The main issue is the formaldehyde contained in many of the products. Formaldehyde can irritate the eyes and nose, cause skin rashes, and cause asthma-like breathing problems. Formaldehyde is also considered a carcinogen by many organizations.
These hair products contain formaldehyde because it makes the product more effective and longer lasting, but there may be a high health cost, especially to the stylists who perform the procedure.
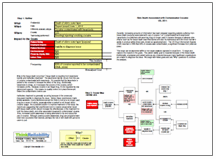
This issue can be analyzed by building a visual root cause analysis called a Cause Map. Click on “Download PDF” above to view a high level Cause Map for this issue.
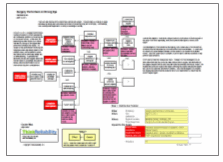
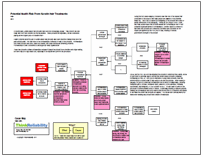 During the root cause analysis, it became clear that one of the causes that contributed to this issue is that many people are unaware of the potential health risk. This in turn is caused by mislabeling of the products and a lack of safety instructions on the packaging. Testing by the Oregon OSHA found that many keratin-based hair products labeled as “formaldehyde free” in fact contained significant levels of formaldehyde. Another cause to consider is that these hair products are considered cosmetics and cosmetics do not require pre-approval by the FDA prior to sale, resulting in minimal government oversight of the product.
During the root cause analysis, it became clear that one of the causes that contributed to this issue is that many people are unaware of the potential health risk. This in turn is caused by mislabeling of the products and a lack of safety instructions on the packaging. Testing by the Oregon OSHA found that many keratin-based hair products labeled as “formaldehyde free” in fact contained significant levels of formaldehyde. Another cause to consider is that these hair products are considered cosmetics and cosmetics do not require pre-approval by the FDA prior to sale, resulting in minimal government oversight of the product.
OSHA and the FDA are both investigating the products to determine their safety, but as of right now it is perfectly legal to sell and use keratin-based products containing formaldehyde in the US. But if you’re interested in using these products, there are several facts you should know to help keep you as safe as possible. When reading a package, it’s good to know that formaldehyde can be listed in multiple ways, including methylene glycol, formalin, methylene oxide, paraform, formic aldehyde, methanal, oxomethane, oxymethylene, or CAS Number 50-00-0. It’s also safer to perform this procedure in a well-ventilated area or outside. Additionally, wearing a mask will prevent inhaling the formaldehyde and some salons now provide them to consumers and stylists to use while the keratin hair products are applied. You should also carefully wash your hands after handling any product that contains formaldehyde.