Recent articles have related several stories of patients being injured or even killed by medical tubing mix-ups. A product used other than intended that results in a patient death is one of the “Never Events” – events that should never happen at healthcare facilities. An article in The New York Times discusses injuries and deaths caused by accidentally connecting food meant for a feeding tube through an intravenous (IV) line. A specific incident mentioned in the article can be analyzed in a Cause Map to capture all of the causes in a simple, intuitive format that fits on one page.
 In this case, a pregnant woman was prescribed a feeding tube to ensure that she and her baby were getting adequate nourishment. The feeding tube was improperly connected to the intravenous (IV) line, causing liquid food to enter her veins, causing sepsis which killed her and her fetus.
In this case, a pregnant woman was prescribed a feeding tube to ensure that she and her baby were getting adequate nourishment. The feeding tube was improperly connected to the intravenous (IV) line, causing liquid food to enter her veins, causing sepsis which killed her and her fetus.
One issue (cause) is that medical personnel made an incorrect connection. Although there was no information given in the article, this would certainly be an area for the responsible organization to look at in more detail and determine if there are steps that can be taken to reduce the risk of these types of errors. (Some organizations have found success with color coding the tubes, for example.)
However, another issue is that the tubes COULD be incorrectly connected in the first place. The number of errors in feeding tube connections (discussed in an article from The Joint Commission Journal on Quality and Patient Safety) has led the U.S. Food and Drug Administration (FDA) to consider declaring these products unsafe.
The tubes become compatible with other tubing connections (such as IV) when needle-free connectors were adopted, to increase caregiver safety (by limiting exposure to needles). Since then, there have been issues with the compatible tubing. (A history of tubing issues is found on the PDF, which can be downloaded by clicking “Download PDF” above.) And, feeding tube connections that are incompatible with other tubing lines are difficult to find. There are many causes given for the delay of developing incompatible tubing, including resistance from the medical industry, difficulties with the FDA approval process, and a delay in forwarding requirements for incompatible tubing. This delay is mainly attributed to waiting for an international group to develop a recommendation regarding tubing, which is expected to take several years.
The FDA has an expedited review process which allows approval of a device if it works like an already approved device, regardless of whether that device is safe, or has been recalled. Because compatible tubing devices have already been approved, new devices that use the same – compatible – connection can go through this expedited process, whereas incompatible connections can not. Without federal agencies requiring change, it’s been difficult getting manufacturers to update their products.
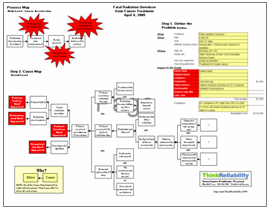
View the problem outline, Cause Map, and timeline of tubing issues by clicking “Download PDF” above.


 The Therac-25 allowed the beam to be turned on without error (minus the overridden warning) in this circumstance. The Therac-25 had no hardware protective circuits and depended solely on software for protection. The safety analysis of the Therac-25 considered only hardware failures, not software errors, and thus did not discover the need for any sort of hardware protection. The reasoning given for not including software errors was the “extensive testing” of the Therac-25, the fact that software, unlike hardware, does not degrade, and the general assumption that software is error-proof. Software errors were assumed to be caused by hardware errors, and residual software errors were not included in the analysis.
The Therac-25 allowed the beam to be turned on without error (minus the overridden warning) in this circumstance. The Therac-25 had no hardware protective circuits and depended solely on software for protection. The safety analysis of the Therac-25 considered only hardware failures, not software errors, and thus did not discover the need for any sort of hardware protection. The reasoning given for not including software errors was the “extensive testing” of the Therac-25, the fact that software, unlike hardware, does not degrade, and the general assumption that software is error-proof. Software errors were assumed to be caused by hardware errors, and residual software errors were not included in the analysis.