Surprisingly, many of what are considered laboratory errors do not actually occur in the lab. But errors related to laboratory testing can negatively impact patient care. We can look at the impacts and causes of errors related to diagnostic testing in a Cause Map, which allows us to visually diagram cause-and-effect relationships.
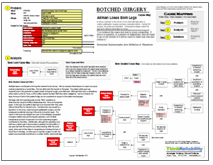
We begin this type of root cause analysis by determining the impacts to the organization’s goals. In this case, because we want to consider all possible sources of diagnostic errors in a proactive analysis, we will look at the generic goals for an organization that provides healthcare. Diagnostic errors can cause an impact to the patient safety goal because of the risk of impact to patient treatment. Employees’ abilities to do their job is impacted because they may be receiving incorrect information from lab testing. There is a risk of impact to the patient’s treatment, which is an impact to the patient services goal. Additionally, there is a risk of performing unnecessary treatment as a result of incorrect testing results, which could impact both the property and labor goals.
 Once we have determined the impacts to the organization’s goals (and there may be more impacts for specific incidents involving diagnostic testing errors), we can ask “Why” questions to determine the causes that result in these impacts. We will begin with the patient safety goal impact. The patient safety goal is impacted because of the risk of an impact to a patient’s treatment. This includes the possibilities of a risk of delayed treatment, risk of not receiving needed treatment, and a risk of unnecessary treatment. Delayed treatment can occur from a delayed diagnosis, which could result from either delayed or incorrect testing results.
Once we have determined the impacts to the organization’s goals (and there may be more impacts for specific incidents involving diagnostic testing errors), we can ask “Why” questions to determine the causes that result in these impacts. We will begin with the patient safety goal impact. The patient safety goal is impacted because of the risk of an impact to a patient’s treatment. This includes the possibilities of a risk of delayed treatment, risk of not receiving needed treatment, and a risk of unnecessary treatment. Delayed treatment can occur from a delayed diagnosis, which could result from either delayed or incorrect testing results.
Delay of testing results can be caused by delayed reporting of results, potentially due to a lack of time requirement for reporting results and/or a lack of tracking these results. A possible solution to delayed reporting of results can be to implement a standardized process for reporting results, which may include time limits or guidelines for reporting results.
Incorrect treatment – whether that is not getting needed treatment or receiving unneeded treatment – can result from an incorrect diagnosis. An incorrect diagnosis can result from an incorrect assessment of diagnostic testing. An incorrect assessment can result from either an incorrect interpretation of laboratory test data or incorrect data from the lab testing.
Incorrect interpretation of lab testing can result from reports that are difficult to interpret, either due to a confusing layout or illegibility. A solution to this is to have a standardized reporting form. Other potential causes of incorrect interpretation include confusion of verbal reporting (such as over the phone) or results not being interpreted by a specialist. Solutions that can reduce this confusion include providing reports electronically when available or repeating results when provided verbally, and making lab experts available for interpretation.
Three main reasons that incorrect data is provided as a result of lab testing is that the specimen is associated with the wrong person, possibly because a patient is misidentified, a specimen is mislabeled, or information is entered incorrectly into the computer. Possible solutions are to use two patient identifiers and label the specimen in the presence of the patient.
Contaminated specimens can also cause incorrect testing results. Specimens can be contaminated at collection, handling, or testing. Any of these issues can be caused by insufficient quality control. The risk of contamination can be minimized by a standardized quality control procedure.
Lastly, incorrect diagnostic data can result from the wrong test being performed. This could occur due to equipment failure, an incorrect entry into the computer, or the wrong test being ordered. More details about any specific incident can be added to the Cause Map based on evidence gathered in the course of an investigation.
To view the Outline and Cause Map, please click “Download PDF” above. Or click here to read more.